Clonal plasma cells in peripheral blood (PB) are crucial for diagnosing plasma cell neoplasm. The diagnostic criteria for plasma cell leukemia (PCL) have recently been revised to 5% or more circulating plasma cells (CPCs) in PB. Considering that CPCs are an independent worse prognostic factor in multiple myeloma (MM), elucidating how many CPCs are sufficient for distinguishing patients between PCL and other plasma cell neoplasm is important yet uncertain. These CPCs have been recently discussed in MM, characterized by more immature phenotype, clonogenic potential, and high expression of cancer stem cell (CTC) markers. Though the exact mechanism of peripheral circulation still needs further studies, MM CTCs are considered subclones of bone marrow (BM) plasma cells.
On the other hand, previous studies regarding PB plasma cells in primary PCL (pPCL) have been mainly focused on similar characteristics to BM plasma cells. Considering significant prognostic differences based on CPCs, PB from PCL patients should have distinct features. Previous studies on PCL revealed that high genetic heterogeneity and accumulation of diverse high-risk genetic abnormalities eventually led to the disease. Therefore, we aimed to investigate the genetic profile of CPCs in PCL to exhibit proper evidence for diagnostic criteria and improve the current practices.
We retrospectively identified patients diagnosed with PCL from 2015 to 2017 at Seoul National University Hospital. Our study population included 4 pPCL and 2 secondary PCL (sPCL) patients. We retrospectively collected patients' clinical information and laboratory test results and performed targeted sequencing using the NGS panel of 647 genes related to hematologic malignancies.
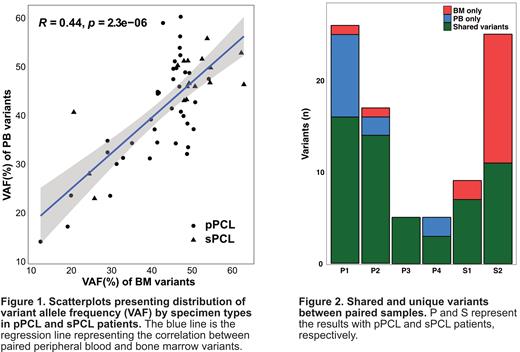
In all analyzed samples, 51 in PB and 40 variants in BM were detected in pPCL. The median number of variants in pPCL patients was 11 per patient (range 5-25) in PB and 10 per patient (range 3-17) in BM. When comparing variant allele frequency (VAF) between the paired samples, there was a moderate correlation (R=0.44, P<0.05). Similarly, a moderate correlation was observed in both pPCL (R=0.41, P<0.05) and sPCL (R=0.47, P<0.05) groups.
When comparing paired variants between PB and BM, 38 were commonly detected in both samples in pPCL patients. Fifteen variants were identified in only one of the two samples, 12 in PB and 3 in BM. Most variants showed a heterogeneous distribution of genes. We checked the paired samples using an Integrative genomics viewer. As a result, twelve pPCL and thirteen sPCL variants were identified in the paired samples. These variants were initially excluded in the filtering process because of low VAF.
Consequently, three unique variants were identified in each pPCL and sPCL group, which were considered truly unique. In pPCL patients, MED12, VPS13B variant from PB, and ZMYM3 variant from BM were identified in one of the paired samples. In sPCL patients, unique variants were identified in ARID1A, FANCE, and TP53. In addition, we determined that PB VAF is higher than BM VAF in shared variants of pPCL patients. Conversely, BM VAF tended to be higher than PB VAF in sPCL. However, plasma cell% was generally higher in BM than PB, regardless of pPCL or sPCL. Therefore, the differences in the percentage of plasma cells between PB and BM samples cannot explain the unique variants detected in only one of the paired samples.
pPCL is an aggressive disease characterized by a markedly high diversity of heterogeneous mutation distribution and high mutational burden. The clinical outcomes of pPCL are still poor in the novel agent era. There is an urgent need to improve the current diagnosis and treatment strategy. As shown in this study, CPCs in pPCL are not simply part of the malignant plasma cells shed from the BM but have characteristics distinct from them. In this study, we exhibited that PB variants had higher VAF than BM variants, and some unique variants were identified only in the PB samples. Conversely, in sPCL, more diverse variants were detected mainly in BM samples, and VAF was also higher in BM than PB. Considering unique variants detected in PB and the high genetic heterogeneity of the disease, PB should be included in the diagnosis of PCL to determine the exact genetic profile.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal